ECCS B310/B320 Electrochemical reaction visualization confocal system
Japan Lasertec Corporation Battery Electric
With the rapid development of technology, we all know that lithium-ion batteries (LIBs) are used in electrical products and electric vehicles for power storage, and the demand for them is likely to increase. However, since lithium is not an abundant metal, it is expensive. On the other hand, sodium is abundant and inexpensive; and interest in sodium ion batteries (SIBs) has been growing.
Materials that have been studied for use as SIB negative electrodes include hard carbon and tin. Hard carbon can be cycled more than 100 times, but the capacity is only about 250 mAh/g. Sodium batteries with hard carbon electrodes are smaller than equivalent lithium batteries. Tin, on the other hand, has a capacity of about 500 mAh/g, but suffers from poor cycling performance (only a few cycles) and volume shrinkage of the tin electrode (about 5.3 times larger than hard carbon) due to large expansion, accompanied by embedding (alloying) and extraction (dealloying) of Na ions. these alloying/de-alloying processes of Sn with Na are similar to the alloying/de-alloying processes of Sn with Li. Therefore, a key factor is the inhibition of the volume change during cycling by the tin-based electrode. As shown in Figure 6 in the original article, the cycling performance of tin electrodes for SIBs can be improved by employing polyacrylic acid (PAA) binders. In this study, the capacity of the Sn electrode was maintained at about 500 mAh/g after 20 cycles. Therefore, the binder is one of the most important constituent materials in the electrode.
LIBs using Sn-Co anodes were first commercialized by Sony Corporation. Sn-Co anodes exhibit good cycling performance because cobalt does not form an alloy with lithium and cobalt buffers the change in electrode volume during cycling. This paper evaluates the properties of Sn-Co electrodes used electrochemically for SIBs to reveal the correlation between cycling performance and binder.
Sn-Co electrodes were prepared using polyvinylidene fluoride (PVdF) or PAA as a binder. The electrochemical properties of Sn-Co electrodes with PAA binder were examined by conducting constant current discharge-charge experiments, and the results were compared with those obtained using PVdF as binder. In addition, the volume change of Sn-Co electrodes with PAA or PVdF during Na ion insertion (alloying)/extraction (de-alloying) was evaluated for the first time by in situ optical microscopy. The crystal structure changes of Sn-Co during cycling were characterized by X-ray diffraction (XRD), and the morphological changes during cycling were analyzed by scanning electron microscopy (SEM) and optical microscopy.
This paper focuses on the electrochemical performance of Sn-Co to show the correlation between the cycling performance and the binder of the electrode assembly material. Compared to polyvinylidene fluoride (PVdF), the Sn-Co electrode of polyacrylic acid (PAA) exhibited better cycling performance (~300 mAh/g for 30 cycles). This better cycling property of PAA is attributed to the slight change in electrode volume during cycling as revealed by in situ optical microscopy. In addition, Na pre-doping in the Sn-Co electrodes increased the average Coulombic efficiency from 95.4% to 99.9% at 2-10 cycles.

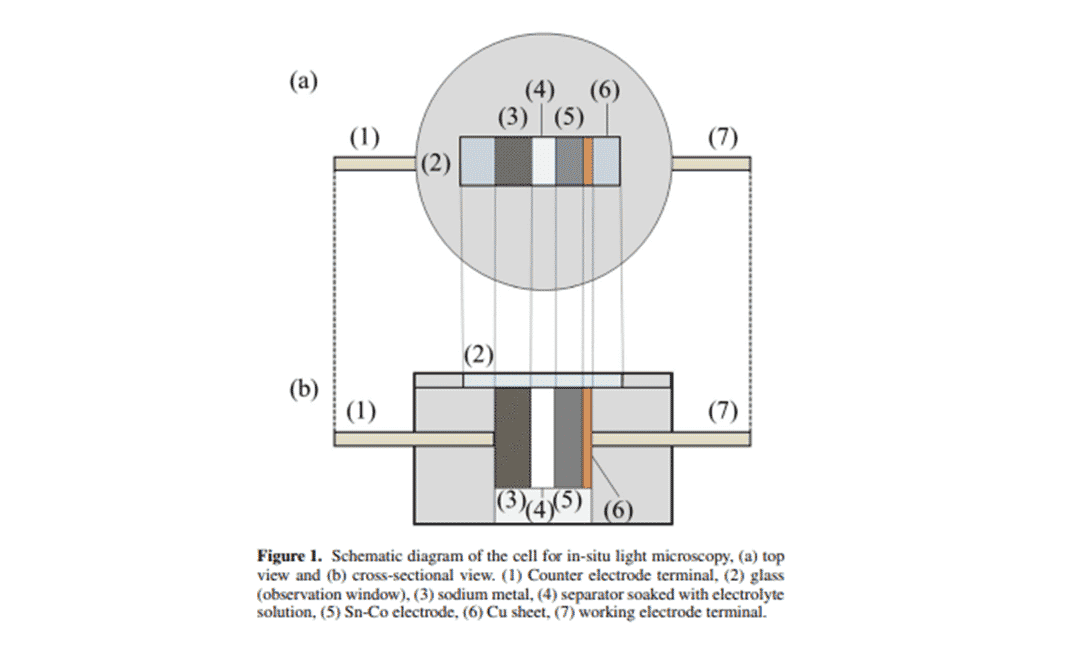
Image 1 shows the change in electrode volume during cycling observed by using an in situ optical microscope (LasertecCorp., ECCSB310). The expansion and contraction of the electrode was estimated in online analysis mode. The circular cell used for in situ optical microscopy was composed of a sodium sheet (0.2 mm thick and 15 mm diameter) counter electrode, an electrolyte solution (1 mol/l NaPF6 EC:DEC 1:1 in volume) with a stacked - soaked polypropylene septum (19 mm diameter) and a Sn-Co (0.02 mm thick and 14 mm diameter) working electrode . The circular cell was cut into a semicircle with the cross section of Na/separator (volume 1 mol/l NaPF6 EC:DEC1:1)/Sn-Co exposed. The cell was then placed in an optical microscope fixture as shown in Figure 1. Discharge-charge tests were performed under the same conditions as for the coin cell and the cross-section was observed through the observation window using an optical microscope.
By Y. Yui, Y. Ono, Hayashi, Y. Nemoto, K. Hayashi, K. Asakura, and H. Kitabayashi
Organization: NTT Energy and EnvironmentSystems Laboratories, Nippon Telegraph and Telephone Corporation, Kanagawa 243-0198, Japan
Published: Manuscript submitted October 21, 2014; revisedmanuscript received December 16, 2014. This paper is part of the FocusIssue of Selected Presentations from IMLB 2014.)
Journal: Journal of The ElectrochemicalSociety, 162 (2) A3098-A3102 (2015)
Article source website.Sodium-Ion Insertion/Extraction Properties of Sn-CoAnodes and Na Pre-Doped Sn-Co Anodes
Japan Lasertec Corporation Battery Electric