ECCS B310/B320 Electrochemical reaction visualization confocal system
Japan Lasertec Corporation Battery Electric
Introduction
With the rapid development of the times, lithium-ion batteries (LIBs) have been widely used in electric and hybrid electric vehicles. However, in order to increasingly increase the output power density, it is necessary to design optimal porous structures in the electrode layers, since lithium ion and electron transfer strongly depend on such heterogeneous structures. In any case, Si and Sn electrodes have been developed as high-capacity anode active materials. In particular, various new materials have been reported from the point of view of capacity, safety and cycling performance. However, few studies have been conducted to design electrode structures with these negative electrode materials because it requires consideration of volume expansion effects during charging and discharging and is difficult. In our previous study, we calculated the electrochemical reactions and mass transfer while using the volume expansion of the active material as a parameter for our calculations. In this study, we first compared the results of this simulation with the actual thickness variations and experimentally obtained charging curves. Next, we used the model to compare each anode active material under charging conditions. We used the previous simulation model. The following assumptions were made: (1) the electrode layer is a homogeneous porous structure, so one-dimensional calculations were performed; (2) the temperature distribution within the cell is uniform; and (3) the particle size of the active material is small, allowing the lithium to diffuse rapidly within the active material particles. Therefore the lithium concentration distribution in the particles is uniform; (4) the positive active material is LiCoO2 and the negative electrode is graphite or silicon. (5) The effect of cracking and flaking from the electrode layer is neglected.
Applications
In this paper, we focus on the fact that in order to improve the cell performance of lithium-ion batteries, not only material development but also the optimal design of the electrode structure is important. However, it is difficult to examine the effect of volume expansion of the anode active material on the internal phenomena, and therefore few studies have been conducted to understand the effect of the optimal electrode layer. In this study, we investigated the effect of the expansion ratio of the active material on the net charge capacity and net charge multiplier performance in the case of graphite and silicon as anode active materials. Charging characteristics were calculated using equations based on porous electrode theory and expressing the parameter changes caused by swelling. In addition, the validity of the simulation model is verified by comparing the thickness variation and charging curves with experimental measurements. As a result, the cell capacity of graphite cells is higher than that of silicon cells under high multiplicity conditions. The dynamic structural properties as an effective ionic conductivity parameter depend on the porous structure, which decreases near the diaphragm and increases the ionic conduction resistance. In the case of Si, the utilization of Si active material is lower than graphite because the reaction is significantly concentrated near the diaphragm.

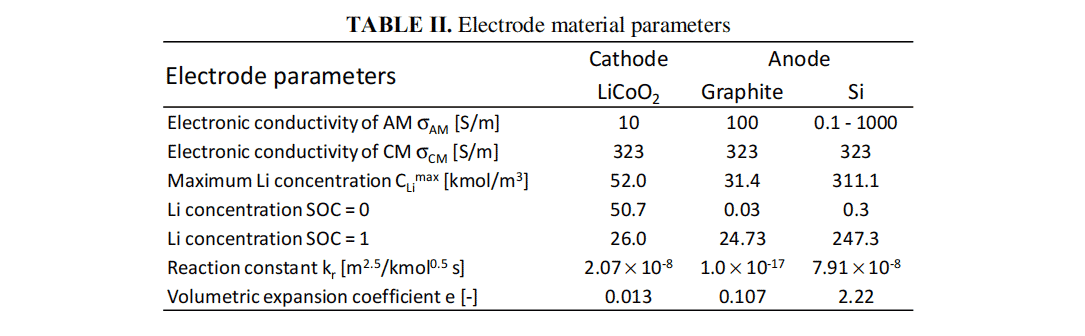
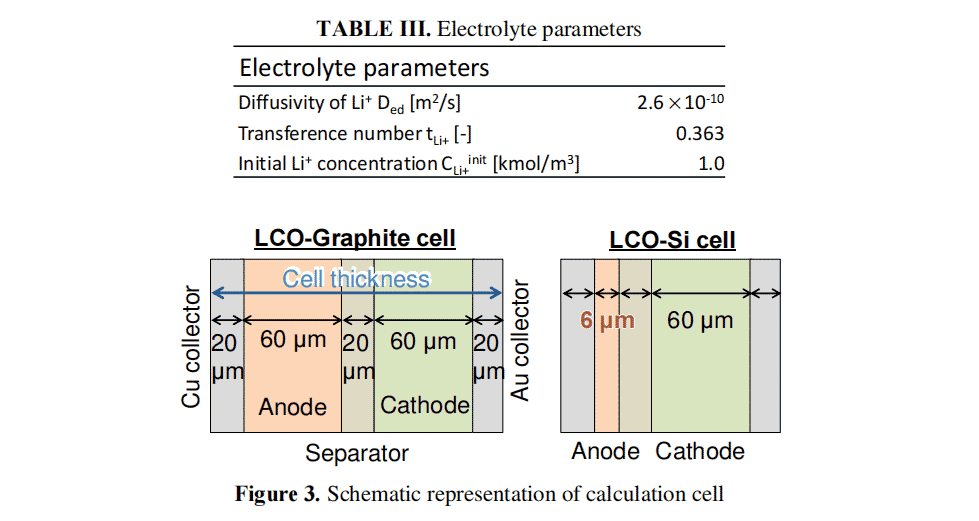
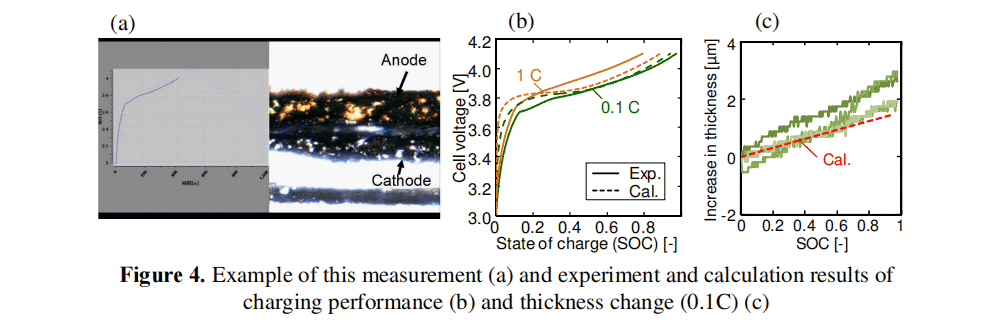
Image 3 presents To confirm the validity of this simulation model, we examined the relationship between charging performance and electrode layer thickness variation. The results were also compared with the simulation results. We used an electrochemical observation system (ECCS B310, Lasertec Corporation, Japan) to observe the cross section of LiBs under charging conditions. Figure 4 shows examples of measured and experimental and calculated results of charging performance and thickness variation. The LCO and graphite electrode layers were used as cathode and anode, respectively. In this comparison, the simulations were performed at a thickness of 40 µm. This is different from Figure 3. In Fig. 4 (a), the cell thickness variation is measured at any four points for each SOC. In these plots, the calculated and experimental results of the charging curve and thickness variation are almost identical to each other. Therefore, the validity of the simulation can be confirmed.
Source
Authors: Gen Inouea , Kazuki Ikeshitab , Minami Iwabub , Yukari Sagaeb and Motoaki Kawase
Institution: Department of Chemical Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan; Department of Chemical Engineering. Kyoto University, Kyotodaigaku Katsura, Kyoto, 615-8510, Japan
Journal: The Electrochemical Society
Article source website.Simulation of Lithium-Ion Battery with Effect of Volume Expansion of Active Materials
Japan Lasertec Corporation Battery Electric